脂质体NMN
NMN,全称β-烟酰胺单核苷酸,是一种自然存在且具有生物活性的核苷酸,是细胞内一种重要的代谢中间产物,也是NAD+合成的直接前体物质,具有多重生理功效。
利用LipoAvail® 脂质体技术将NMN包封于类脂质双分子层内。
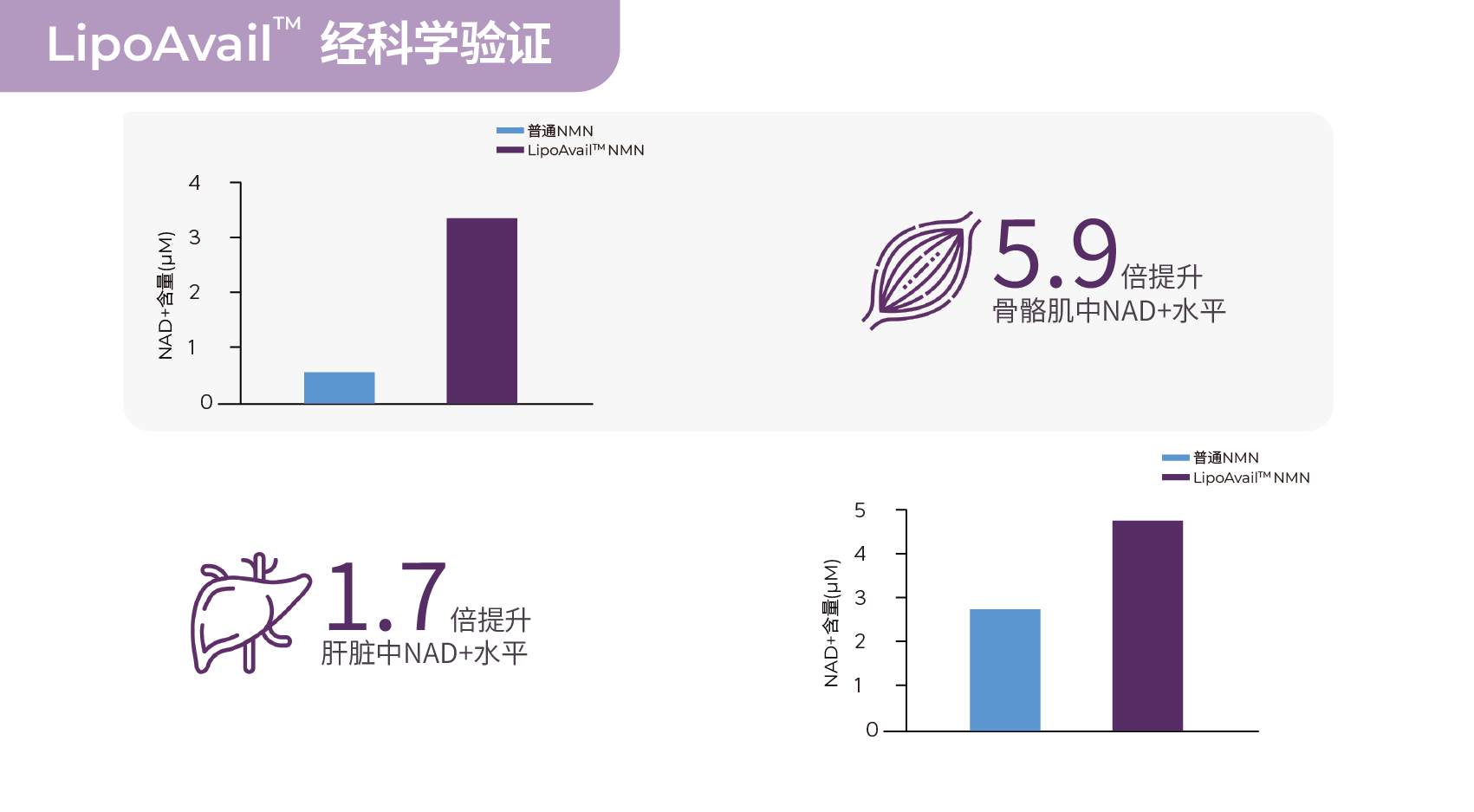
相比NAD+,LipoAvail®脂质体NMN提升骨骼肌中的NAD+的含量约6倍。
-
支持健康衰老

-
保护心脏

-
提升认知功能

-
改善抑郁情绪

-
体重管理支持



NMN效果再进阶,开启差异化竞争新局面
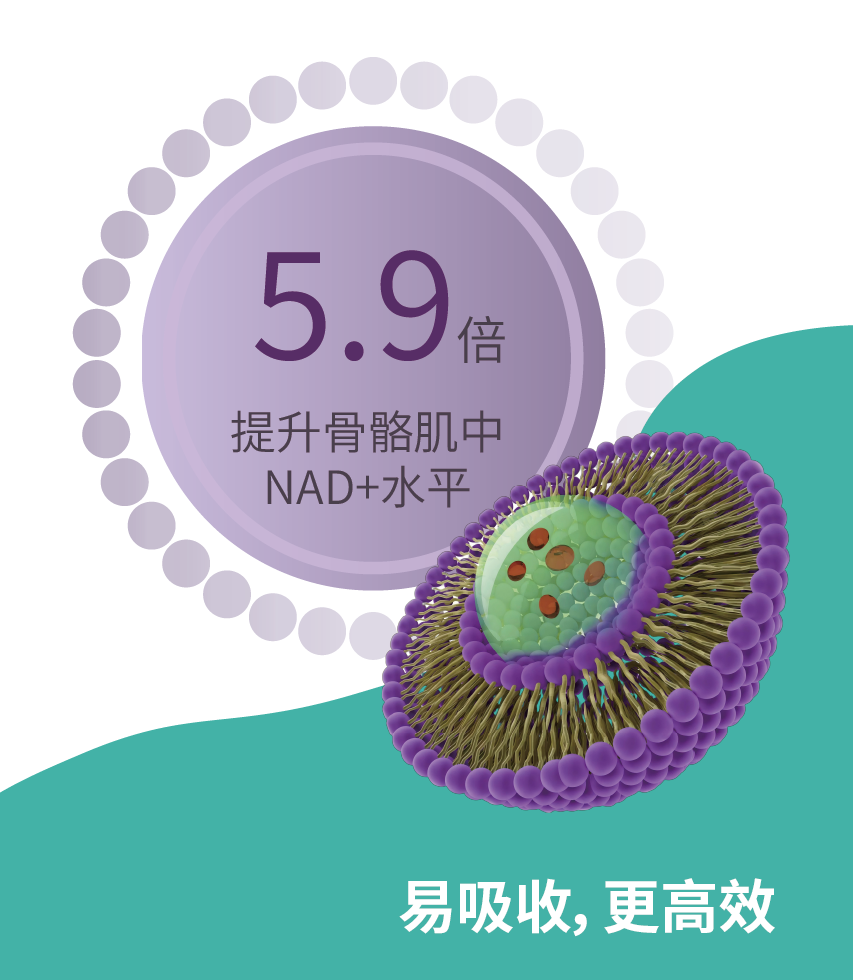
相比NMN,LipoAvail® 脂质体NMN提升骨骼肌中NAD+水平5.9倍
LipoAvail® 脂质体NMN提升肝脏组织中的NAD+水平约1.74倍
卓越的理化性质
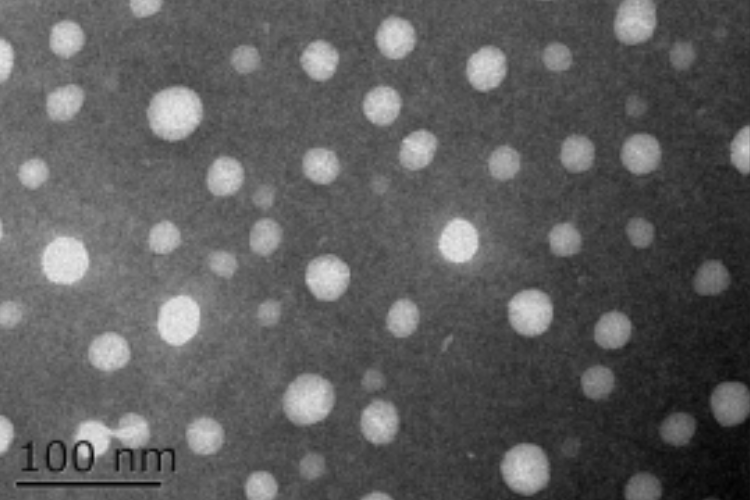
TEM下的粒径分布
NMN的含量为50%、70%
规则的球形性状,
良好的粒径分布,平均粒径为30 nm
良好的应用性
-

粉剂
-

片剂
-

硬胶囊
-

软胶囊
第三方品质认证
小样
*上述声明未经过国家权威机构评估。本产品不用于诊断、治疗、治愈或预防任何疾病。
仅供公司之间交流不直接针对最终消费者。
如需相关文献,请联系我们。
本网站为音芙医药科技(上海)有限公司的官方中文站点。特此声明,本网站所展示的部分产品可能尚未获得中国境内的销售许可,因此并不在中国市场进行销售。用户在使用本网站信息时,请自行判断信息的适用性,并遵守所在国家或地区的法律法规。
LipoAvail®是音芙医药科技(上海)有限公司的商标。